I feel like I’m at an addiction meeting.
My name is Brennan Koch, and I don’t use the swap and drop method to write formulas for ionic compounds.
There. I said it. Over the years I have vacillated between teaching the students to use it and not. I have often used it as a backup for kids that were struggling. What I have found is that kids that use the swap and drop method because they are struggling tend to continue struggling.
I now use the least common multiple (LCM) method. While the reference to math class can tend to freak some kids out at first, I believe that it helps remove some of the common errors seen in swap and drop.
Mindless vs Mindful
One thing I don’t like about swap and drop is the mindlessness of the activity. Many students have no idea why they are swapping and dropping. They just do it because that is what they do. I find that there is a slightly higher level of understanding about the purpose of the subscripts using the LCM method. The line of questioning goes something like this for iron (III) oxide.
What is the charge on the oxide?
2-
What is the charge on the iron ion?
3+
Do those charges cancel each other out to zero?
No.
How are you going to make those charges equal one another?
I’m not really sure.
What is the least common multiple of 2 and 3.
6
So what you are saying is the total negative charge has to be six and the total positive charge has to be six. What would you need to do to this formula to make those ions cancel each other?
I would need two irons and three oxygens.
Perfect! Write that down.
I like the fact that the LCM multiple method creates a mindset for balancing charges over the mindlessness of moving numbers around arbitrarily. One of the errors that has decreased dramatically since moving away from swap and drop is the negative subscript. All too often, kids would bring down the negative sign with the charge and leave it. Even though I would try to explain why having -2 iron ions isn’t very realistic, it didn’t matter in their brain. They had swapped and dropped. LCM reduces this error as they are more purposefully trying to cancel charges. (Though it still shows up occasionally)
Reduction in Empirical Formula Errors
Since ionic compounds don’t make molecules, the formula is simply stating the ratio of cations to anions. The swap and drop method tends to overlook this idea. Classic examples occur with compounds like magnesium oxide and lead (IV) carbonate. I know that you teach your students to reduce the subscripts on magnesium oxide, but we don’t often get to teach the why. When you are using the LCM method and asking kids what needs to be done to cancel out charges, the error of bringing down unnecessary subscripts decreases.
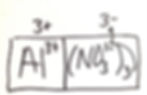
When balanced charges are the mental goal, students are more likely to write correct empirical formulas. When I am drawing this on the board, I have a structure that I like to use to help the students visualize the total charge on the cations and anions.

I put a box around the anion and a box around the cation. The first thing the I do is have the students figure out the LCM and then write the positive and negative version of that number above the box. This is the total ion charge. The students now have to add the correct subscripts to make the total charges cancel each other. They quickly learn to do this and place the correct subscripts.

I teach that subscript times ion charge equals total ion charge. The ultimate goal is to have equal magnitudes of total ion charge.
Is this more work initially than swap and drop? Of course it is. But I feel that the extra effort up front leads to a more complete understanding as they continue through chemistry.
LCM Reduces Polyatomic Error Mistakes
Tell me if you have seen this error in swap and drop. Aluminum nitrate. The kids write down AlNO3 they look down and see the one and the three already swapped and dropped and so they leave it. LCM forces the students to box the entire polyatomic ion and ask how many of those do I need to cancel out the cation. This leads to a higher success rate as well as avoiding those silly errors with polyatomics. Subscript times ion charge equals total ion charge. I repeat and repeat that phrase.

LCM Gets Rid of the Ambiguity
When using the LCM, many kids will figure out swap and drop on their own. They ask a pertinent question.
Can’t I just bring the numbers down and swap them?
And your answer has to be, “Yes… most of the time.”
One of the challenges of chemistry is the “most of the time” phrases. It makes kids wary. If you use the LCM you can confidently say if the charges cancel out using the LCM then the formula is right.
We tell the kids enough little white lies to make their lives easier as it is. The octet rule. S and p are the only valence. All gases behave ideally. Soluble and insoluble compounds are actually soluble and insoluble. The list goes on forever. We can take the idea that swap and drop always works off this list. Just don’t teach it.
I’m not trying to change your swap and drop religion like an uninvited guest knocking on your door and wanting to spread the good news of LCM. But if this conversation makes you think about why you teach what you teach, then my mission is complete.
Need some concentrated practice writing ionic formulas? Try CHeMgO! This bingo-style classroom game gives students opportunities to practice writing ionic formulas in a competitive environment. Plus you can choose the level of challenge appropriate for that class with no extra effort. Every card has four levels of challenge; binary, binary with Roman numerals, add in polyatomics, all of the above plus acids. Give it a shot.

